Research Report
Distribution and Sources of Fatty Acids in Sediment Samples from Shatt Al-Arab Estuary and Northwest Arabian Gulf 
 Author
Author  Correspondence author
Correspondence author
International Journal of Marine Science, 2015, Vol. 5, No. 40 doi: 10.5376/ijms.2015.05.0040
Received: 30 May, 2015 Accepted: 18 Jun., 2015 Published: 19 Jun., 2015
Al-Timari A.Ak., Al-Saad H.T , Douabul A.A., Salah. M., and Hantoosh A.A,.2015, Distribution and sources of fatty acids in sediment samples from Shatt Al-Arab estuary and Northwest Arabian Gulf, International Journal of Marine Science, 5(40): 1-6 (doi: 10.5376/ijms/2015.05.0040)
Surface sediments (10 cm) of the subtropical Shatt Al-Arab estuary and Northwest Arabian Gulf were collected to study the fatty acids distribution and sources .Samples were analyzed by gas chromatography. Other relevant parameters were measured including total organic carbon, and grain size. A total of 31 fatty acids were identified from the superficial sediment samples, including polyunsaturated PUFA, monounsaturated (MUFA), and saturated and branched fatty acids. The total FA (TFA) concentrations were in the range 13.32-35.72 µg/g dry weight (dw).Generally, the abundances of the 31 FAs showing strong even/odd numbered predominance, and ranging from C12 to C32 were C14 (1.68-12.45%), C16 (2.92-13.49%), C18 (0.87-12.75), C19 (0.87-3.29%), C24 (0.19-6.99%) and C26 (nd-8.90%), C28(nd-5.91%). These distributions are indicative of input of terrestrial, phytoplanktonic and bacterial lipid residues. The sums of long chain FAs (LCFAs) C24-C32 concentrations of were calculated. High values of terrestrial Organic Matter (OM)were found at Shatt Al-Arab, the values were in the range 4.05-4.46 µg/g (dw), with contribution relative to the TFAs ranging from 12.49-21.99% (mean 17.99%). While low values were found at the North West Arabian Gulf , the values were in the range 0.03-0.09 µg/g (dw), with contribution relative to the TFAs ranging from 0.19-0.68 % (mean 0.36%).
Introduction
Knowledge of the source and reactivity of Organic Matter (OM), as well as factors controlling its distribution, is important to understand the role of estuarine and coastal systems in global biogeochemical cycles. Rivers are an important interface between the continents and oceans since they are involved in the delivery a key flux of organic compounds (autochthonous and allochthonous) to the ocean, rapidly depositing them on shelve sand margins (Saliot et al., 2001). Lipid classes have been used as indicator for understanding the origin and source of organic matter (Camacho-Ibar et al., 2003; Oyo-Ita et al., 2010).
Several studies of the OM composition of subtropical rivers have shown that much of the transported material is predominantly derived from highly degraded soil material (Hu et al., 2006). Therefore, tracing OM sources in sediments has received considerable attention due to the importance of understanding OM cycling in the aquatic environment (Jaffe et al, 2006).
Besides being the primary membrane and energy storage in planktons, fatty acids (FAs) play important role in source characterization of OM in sediment (Aboul-Kassim and Simoneit, 1996; Fahl and Stein, 1999; Al-mutlaq et al., 2008). However, their utility as quantitative tracers of the different organic carbon sources is complicated by many factors: ambiguities in organic matter source are common due to the fact that individual Fatty Acids (FAs) may differ in their digenetic susceptibility during particle settlement through the water column (Wakeham, 1999; Mudge, 2005). Other factors that affect individual FAs include water temperature (Fileman et al., 1998), dissolved oxygen concentration (Harvey and Marko, 1997) and degree of sorption onto mineral matrices (Nissemann and Schubert, 2006).
Concentration of fatty acids from the sediments of Hor Al-Hammar marshes, Shatt Al-Arab and North-West Arabian Gulf have been studied (Al-Timari and Al-Saad, 1990; Al-Saad and Al-Timari, 1993). Recently Al-Saad et al. (2009 ; 2010) studied the seasonal variations of dissolved and particulate fatty acids in water of Shatt Al-Arab estuary and North-West Arabian Gulf. Due to the lack of information on the fatty acids compositions in sediments from the Shatt Al-Arab estuary and North-West Arabian Gulf, we study the distribution and sources of the Fatty Acids in sediment of these area and compare the results with those for other shallow and deep aquatic sedimentary environments.
Materials and Methods
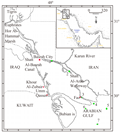
Sediment samples were taken by means of Van Veen grab sampler from seven stations in Shatt Al-Arab River and North-West Arabian Gulf (Figure 1).Samples were stored at appropriate tem till further use For the study of fatty acid, the sediment samples were freeze dried, ground and passed through a 1mm metal sieve. Fifty gram of dry homogenous sediment was Soxhlet extracted for 24 h with 200ml of a mixture of methanol and benzene (1:1) (Goutx and Saliot,1980). At the end of this period, the extract was reduced in volume to approximately 10 ml using a rotary evaporator. Then the extract was saponified for 2 h with a solution of 4 N KOH in methanol:benzene mixture (1:1). After extracting the unsaponified matter with hexane, the extract was dried over Na2SO4, the concentrated, treated with copper to remove sulfur, and further concentrated by a stream of N2. The concentrated extract was cleaned up by a column chromatography on 5% deactivated florisil. An aliphatic fraction was eluted with 50 ml of hexane. The saponified material was used to release free fatty acids by acidifying with 6N HCl and extracted with ether. The fatty acid extracted was then methylated by a solution of 14% BF3 in methanol according to Metcalfe and Schmitz (1961). Quantitative and qualitative analyses of compounds were performed on a Perkin-Elmer sigma 300 capillary gas chromatography with splitless injection flame ionization detector using SP2100, WCOT column 25 m with He as carrier gas (1.5 ml/min) , temperature was programmed from 50℃ for 2 min to 300℃ for 30 min at rate 4℃/min. Recovery efficiency of sediment samples exceeded 90% for all compounds. Total Organic Carbon (TOC) was determined by treating subsamples with phosphoric acids to remove carbonates, then dried at 60℃ to constant weight and combusted using a Perkin-Elmer model 240B Elemental analyzer. Grain size analysis was done by using 15 g dried sample, Then wet sieving was carried by sieve of 230 mesh (Folk, 1974). The fine grain (<63 µm) were passed through the Sedigraph ET-5000 instrument.
Results and Discussion
TOC content for the sediments ranged from 0.38 to 0.85% with a mean value of 0.60%. The highest TOC content was measured at station 2 (0.85%) followed by station 3 (0.72%) with the lowest value recorded at station 7(0.38%) (Table 1).
Grain size distribution of the sediment also plays an important role in determining TOC. For instance, fine-grained sediments are reported to adsorb OM more than coarse sandy types with larger grain size (Hyun et al., 2002). In our study the sediments are mainly predominantly of silty clay fraction except station 5 is predominant of sand-silt-clay (Table 1 and Figure 2).
The TOC content of the sediments is typical of a coastal environment exhibiting high TOC contents for samples with high proportion of clay/silt fraction, whereas samples with high proportion of sandy fraction exhibited low TOC ( Al-Timari et al., 2014).
Unlu and Alpar (2006) reported a lower TOC content (ca. 0.07-3.05 %) in surface sediments from Germlik Bay, Turkey where higher TOC values were observed in sediments from a deep trough area than those from shallow areas. Furthermore, much lower TOC content of range 0.32-0.95% was reported for Yangste River with comparable water depth to that of the study area, highlighting the importance of water depth as a factor that determines organic carbon accumulation in aquatic sediments. In addition, variation in the quality and quantity of OM input has been attributed to the varied TOC contents of coastal sediment from Egypt (ca. 1.0-9.1%; Aboul- Kassim and Simoneit, 1996) and surface sediments from Pulau, Tinggi, Malaysia (ca. 2-13%; Humrawali, 2009).
A total of 31 fatty acids were identified from the superficial sediment samples, including polyunsaturated (PUFA), monounsaturated (MUFA), and saturated and branched fatty acids (Figure 3) .The total FA (TFA) concentrations were in the range 13.32-35.72 µg/g (dw) (Table 2) lower than the values for the top section of cores from the Hor Al-Hammar marsh, southern of Iraq, which was in the range 30.44-42.25 µg/g (dw) (Al-Timariet al., 2014), most likely due to the anoxic condition of the water column and greater primary productivity, and is in agreement with numerous studies which provide evidence that shallow water depths (< 50 m) favors' high TFA concentrations in surface sediments (Nissemann and Schubert, 2006; Rushdi et al., 2009). Generally, the abundances of the 31 FAs showing strong even/odd numbered predominance, and ranging from C12 to C32 were C14 (1.68-12.45%), C16 (2.92-13.49%), C18(0.87-12.75), C19 (0.87-3.29%), C24 (0.19-6.99%) and C26 (nd-8.90%), C28 (nd-5.91%). These distributions are indicative of input of terrestrial, phytoplanktonic and bacterial lipid residue (Figure 3 and Figure 4), ( Al-Timari et al., 2014).
Phytoplankton and zooplankton are in particular the most important sources of monounsaturated FAs, and polyunsaturated FAs (PUFAs) with 20 and 22 carbon atoms (Parrish et al., 2000; Zimmerman and Canuel, 2001). In most phytoplanktons, the compositions of unsaturated FA are more abundant than their saturated counterparts except for the population of Cyanophyceae (Lauther et al., 1993). And in most cases the values of the total unsaturated FA to the total saturated FA (U/S) ranges between 1.07 and 1.97 where the Cyanophyceaeare not the majority. Our results showed U/S values < 0.65 for the entire samples, suggesting minor contribution of phytoplankton to the total fatty acid content (Table 2). At the level of monounsaturated FA, C16:1 and C18:1 clearly predominate the other monoenes in most phytoplankton, particularly where Bacillaryoph-yceae population is poorly represented (Lauther et al., 1993).
Claustre et al., (1989) proposed that a diatom source in aquatic sediments may be determined by way of the 16:1/16:0 FA ratio and the sum of all FAs having 16 carbon atoms to the sum of all FAs having 18 carbon atoms (ΣC16/ΣC18). These authors postulated that increased values of these ratios represent increased proportions of diatoms, but due to the higher susceptibility of unsaturated fatty acids to the biological and chemical degradation of living phytoplankton during the sedimentation, the ratio of 16:1/16:0 in surface sediments is usually well below 1 (Birgel et al., 2004). The ratios of our samples were in the range of 0.53 to 1.28, averaged 0.7 except St.7 indicate high value (Table 2).
The PUFAs normally associated with phytoplankton, for example, 20:5ω3 and 16:4ω1, are typical diatom fatty acids (Colombo et al., 1996); 18:2ω6, 18:3ω3 and 18:3ω6 have been used as markers of green macroalgae (Meziane and Tsuchiya, 2000); 22:6ω3 usually indicates dinoflagellate origin (Colombo et al., 1996; Budge and Parrish, 1998; Carrie et al., 1998). However, none of these PUFAs except 18:2ω6 were detected in our samples, because PUFAs are losses by bacterial degradation and/or via zooplankton grazing (Hu et al., 2006). Thus, these PUFAs associated with phytoplankton would not necessarily be expected to be preserved in their original amounts, (Carrie et al., 1998). Moreover the Ratio values of unsaturated and saturated FAs of various carbon numbers (e.g. 18:1/18:0) have also been used to indicate an input from fresh OM. In our samples, low values of this ratio were found (Table 2), suggesting that most of the OM flux to the sediment at the time of sampling may be oxidized Therefore, it is not possible to easily assess the source phytoplankton community structure by fatty acids in sediments (Carrie et al., 1998).
Bacteria are typical sources of odd and branched chain FAs especially the iso- and anteiso-acids, i15:0, i17:0 and a17:0 have been reported by several researchers (Harvey and Marko, 1997). Other FAs reported to be produced by both aerobic and anaerobic bacteria are 15:0 and 17:0 (Parish et al., 2000). The sum of these FAs has been used to estimate the bacterial contribution to reveries and estuarine systems (Harvey and Marko, 1997; Parish et al., 2000).
The sediment of the area were anoxic (Al-Saad and Al-Timari, 1993), Gong and Hollander (1997) were able to trace higher contribution of bacterial FAs in sediments from anoxic deposition than in oxic sediments from the periphery of the Santa Monica Basin. Moreover Analysis of soil OM (SOM) by (Nierop et al. 2003) showed the presence of 19:0 acid reported to be derived from bacteria rather than terrestrial sources. Also Oyo-Ita and Oyo-Ita (2012) observed that the levels of 19:0 acids were high in samples collected near oil spills. Therefore our calculation of the levels of bacterial contribution was based on sum of i15:0, a15:0, i15:0, i17:0, a17:0, 17:0 and 19:0 FAs, the levels were found to be in the range of 19.98% and 33.48% with a mean value of 25.30% which is highest comparison with other contribution at the study area (Figure 5)
 Figure 5 Percentage of MUSFA (planktonic acids), Branch C15 and C17 (iso and anteiso) and C19 (bacterial acids) and the sum of LCFA (terrigenous acids) |
In many aquatic environments, there is interest in determining the extent of contribution from terrestrial plants. Even numbered, saturated long-chain FAs >C24 in aquatic sediments are typically associated with an input of OM from terrestrial higher plants (Parish et al., 2000; Camacho-Ibar et al., 2003; Al-mutlaq et al., 2008).The sum of C24-C32 (ΣC24-C32) concentrations of long chain FAs (LCFAs) were calculated (Table 2) .High values of terrestrial OM (ΣTerri) were found at Shatt Al-Arab (St.1-4 ),the values were in the range 4.05-4.46 µg/g (dw), with contribution relative to the TFAs ranging from 12.49-21.99% (mean 17.99%; Table 2) While low values were found at the North West Arabian Gulf (St.5,6 and 7), the values were in the range 0.03-0.09µg/g (dw), with contribution relative to the TFAs ranging from 0.19-0.68 % (mean 0.36%).
Alternatively, the 18:2 ω6 and 18:3ω3 FAs found to be elevated in most terrestrial plants may be used as terrestrial markers from an examination of terrestrial plant samples (Al-mutlaq et al., 2008). According to these authors, an arbitrary threshold of 2.5% relative to TFAs has been assigned to these indicators. On this basis, samples with values above this may be considered to have a significant terrestrial OM contribution. Our results for these markers are in agreement, showing values >2.50% at most stations.
Conclusions
1- The relatively low TOC content determined for the Shatt Al-Arab estuary and north west Arabian Gulf sediments may be due to its characteristic.
2- The distributions pattern of total fatty acids in superficial sediments and TOC contents are similar, indicating fatty acids are simply associated with the TOC contents of the sediments.
3- Fatty acid composition in superficial sediments and principal component analysis of these data show that the fatty acid lipids mainly derived from phytoplankton, bacteria and terrestrial organic matter in the subtropical Shatt Al-Arab estuary and north west Arabian Gulf.
4- The contribution of planktons to the river, though minor, could not be estimated because of the possible influx of compounds derived from an unspecified terrestrial source.
5- The distribution of total fatty acids and bacterial fatty acids is quite similar, as well as the highest percentage of bacterial fatty acids comparison with other contribution occurs at study area suggesting that bacteria may be a significant component of the fatty acids of these sediments.
6- The highest levels of terrestrial fatty acids and % terrestrial fatty acids at the Shatt Al-Arab indicate freshwater runoff was delivering terrestrial plant material into the estuary.
References
Aboul-Kassim T. A. T., and Simoneit B. R. T., 1996, Lipid geochemistry of surficial sediments from the coastal environment of Egypt: I-Aliphatic hydrocarbon characterization and sources, Marine Chemistry, 54,135-158
http://dx.doi.org/10.1016/0304-4203(95)00098-4
Al-mutlaq K. F., Standley L. J., and Simoneit B. R. T., 2008, Composition and sources of extractable organic matter from a sediment corer in Lake Kivu, East African rift valley, Applied Geochemistry, 23: 1023-1040
http://dx.doi.org/10.1016/j.apgeochem.2007.07.013
Al-Saad, H.T. and Al-Timari, A.A., 1993, Sources of Hydrocarbons and Fatty acids in sediments from Hor Al-Hammar Marsh, Shatt Al-Arab, N.W. Arabian Gulf, Mar. Poll. Bull, 26(10): 559-569
http://dx.doi.org/10.1016/0025-326X(93)90406-A
Al-Saad, H.T., Hantoush, A.A., Mahdi, E.A. and Saleh S.M., 2009, Distribution and sources of dissolved fatty acids in water of Shatt Al-Arab estuary and Northwest Arabian Gulf, Mesop. J. Mar. Sci, 24 (1): 25-34
Al-Saad, H.T. ,Hantoush, A.A., Al-Hello1, M.A., and. Zukhair, M.K., 2010, Seasonal variations of particulate fatty acids in waters of Shatt Al-Arab River and northwest Arabian Gulf.Mesopot, J. Mar. Sci, 25 (1): 41-52
Al-Timari, A.A. and Al-Saad, H.T., 1990, Fatty acids in recent sediments of the marshes, Southern of Iraq, Marina Mesopotamica, 5(2): 325-336
Al-Timari, A.A., Douabul, A. A., Hantoosh, A.A. and Al-Saad, H. T., 2014, Distribution and sources of fatty acids in sediment cores from Al-Hammar marsh, southern Iraq, Marsh Bulletin, 9 (1):23-37
Birgel, D., Stein, R.,and Hefter, J., 2004, Aliphatic lipids in recent sediments of the Fram Strait/Yermak Plateau (Arctic Ocean): composition, sources and transport processes, Marine Chemistry, 88: 127-160
http://dx.doi.org/10.1016/j.marchem.2004.03.006
Budge, S.M. and Parrish, C.C., 1998, Lipid biogeochemistry of plankton, settling matter and sediments in Trinity Bay, Newfoundland. II. Fatty acids, Organic Geochemistry, 29: 1547-1559
http://dx.doi.org/10.1016/S0146-6380(98)00177-6
Camacho-Ibar, V. F., Aveytua-Alczar, L., and Carriquiry, J. D.,2003, Fatty acid reactivates in sediment cores from the Northern Gulf of California, Organic Geochemistry, 34: 425-439
http://dx.doi.org/10.1016/S0146-6380(02)00211-5
Carrie, R.H., Mitchell, L.and Black, K.D., 1998, Fatty acids in surface sediment at the Hebridean shelf edge, west of Scotland, Organic Geochemistry, 29: 1583-1593
http://dx.doi.org/10.1016/S0146-6380(98)00160-0
Claustre, H., Marty, J., Cassiani, L., and Dagaut, J., 1989, Fatty acid dynamics in phytoplankton and microzooplankton communities during a spring bloom in the coastal Ligurian Sea: ecological implications, Marine Microbial Food Webs, 3: 51-53
Colombo, J.C., Silverberg, N. and Gearing, J.N.,1996, Lipid biogeochemistry in the Laurentian Trough I. Fatty acids, sterols and aliphatic hydrocarbons in rapidly settling particles, Organic Geochemistry, 25:211-225
http://dx.doi.org/10.1016/S0146-6380(96)00115-5
Fahl, K. and Stein, R., 1999, Biomarkers as organic-carbon-source and environmental indicatots in the Late Quarternary Arctic Ocean: problems and perspectives, Marine Chemistry, 63: 293-209
http://dx.doi.org/10.1016/S0304-4203(98)00068-1
Fileman, T. W., Pond, D. W., Barlow, R. G. and Mantoura, R. F. C., 1998, Vertical profiles of pigments, fattyacids and amino acids: evidence for undegraded diatomaceous materials sedimenting to the deep ocean in the Bellingshausen Sea, Antarctica, DeepSea Research, 45:333-346
http://dx.doi.org/10.1016/S0967-0637(97)00824-8
Folk, R.L., 1974, Petrology of sedimentary rocks, Hemphill publication Co., Austin, Texas
Goutx, M. and Saliot,A., 1980, Relationship between dissolved and particulate fatty acids and hydrocarbons, chlorophyll a and zooplankton biomass in Villefrache Bay, Mediterranean Sea, Mar. Chem, 8: 299-318
http://dx.doi.org/10.1016/0304-4203(80)90019-5
Gong, C., and Hollander, D. J., 1997, .Differential contribution of bacteria to sedimentary organic matter in oxicand anoxic environments, Santa Monica Basin, California, Organic Geochemistry, 26: 545-563
http://dx.doi.org/10.1016/S0146-6380(97)00018-1
Harvey, R. H., and Marko, S. A., 1997, Kinetics of phytoplankton decay during simulated sedimentation:changes in lipids under oxic and anoxic conditions, Organic Geochemistry, 27:129-140
http://dx.doi.org/10.1016/S0146-6380(97)00077-6
Humrawali, N., 2009, Fatty alcohols in surface sediments from PulauTinggi, Johor, Malaysia, EuropeanJournal of Scientific Research, 32: 419-429
Hu, J., Zhang, H. and Peng, P., 2006, Fatty acid composition of surface sediments in the subtropical Pearl River estuary and adjacent shelf Southern China, Estuarine, Coastal and Shelf Science, 66 :346-356
http://dx.doi.org/10.1016/j.ecss.2005.09.009
Hyun, J. H., Ju, S. j. and Harvey, H. R., 2002, Faecal contamination associated with local reclamation activity inthe Han River Estuary. Journal of the Korean Society of Oceanography, 37: 1-8
.Jaffe, R., Rushidi, A. I., Medeiros, P. M., and Simoneit, B. R. T., 2006, Natural Product biomarkers as indicatorsof sources and transport sedimentary organic matter in a sub tropical river, Chemosphere, 64: 1870-1884
http://dx.doi.org/10.1016/j.chemosphere.2006.01.048
Lauther, A. M., Henri, C., Filbert, M., and Jean, L., 1993, Variations in fatty acid composition of phytoplanktonicproduction in sewage treatment ponds at Meze.Effect on the valorization of microalgal biomass.Biochemistry and Physiology, 104: 769-773
Metcalfe, L.D. and Schmitz, A.A.,1961, The rapid preparation of fatty acid esters for gas chromatographic analysis, Ana. Chem, 33: 363-364
http://dx.doi.org/10.1021/ac60171a016
Meziane, T., and Tsuchiya, M., 2000, Fatty acids as tracers of organic matter in the sediment and food web of a mangrove/intertidal flat ecosystem, Okinawa, Japan, Marine Ecology Progress Series, 200: 49-57
http://dx.doi.org/10.3354/meps200049
Mudge, S. M., 2005, Fatty alcohols- a review of their natural synthesis and environmental distribution, The Soap and Detergent Association, 132: 1-141
Nierop, K. G. J., Naafs, D. F. W., and Eglinton, T. I., 2003, Occurrence and distribution of ester-bound lipids inDutch costal dune soils along a pH gradient, Organic Geochemistry, 34:719-731
http://dx.doi.org/10.1016/S0146-6380(03)00042-1
Nissemann, J., and Schubert, C. J., 2006, Fatty acid biogeochemistry of sediment from the Chilean coastalupwelling region : sources and diagenetic changes, Organic Geochemistry, 37: 626-647
http://dx.doi.org/10.1016/j.orggeochem.2005.11.004
Oyo-Ita, O. E., Ekpo, B. O., Oros, D. R., and Simoneit, B. R. T., 2010, Occurrence and sources of triterpenoidsmethyl ethers and acetates in sediments of the Cross River System of Southeast Nigeria, International Journal of Analytical Chemistry, 14: 21-28
Oyo-Ita,O. E.and Oyo-Ita,I.O., 2012, Fatty Acid and Alcohol Distributions and Sources in Surface Sediments of Imo River, Southeast Niger Delta, Nigeria, Environment and Natural Resources Research, 2 (4): 101-113
http://dx.doi.org/10.5539/enrr.v2n4p101
Parish, C. C., Abrajano, T. A., Budge, S. M., Helleur, R. G., Hudson, E. D., Pulchan, K., and Ramos, C.,2000, Lipid and phenolic biomarkers in marine ecosystems: Analysis and Applications, TheHandbook of Environmental Chemistry, 5:194-223
http://dx.doi.org/10.1007/10683826_8
Rushdi, A. I., Kassim, T. A., and Simoneit, B. R. T., 2009, Organic tracers in sediment from the coastal zone of Ras Abuel-Darag, Environmental Geology, 58: 1675-1687
http://dx.doi.org/10.1007/s00254-008-1668-3
Saliot, A., Mejanelle, L., Scribe, P., Fillaux, J., Pepe, C., Jabaud, A., and Dagaut, J., 2001, Particulate organiccarbon, sterols, fatty acids and pigments in the Amazon Rivers system, Biogeochemistry, 53:79-103
http://dx.doi.org/10.1023/A:1010754022594
Unlu, S., and Alpar, B., 2006, Distribution and sources of hydrocarbons in surface sediments of Gemlik Bay (Marmara Sea, Turkey), Chemosphere, 64: 764-777
http://dx.doi.org/10.1016/j.chemosphere.2005.10.064
Wakeham, S. G., 1999, Monocarboxylic, dicarboxylic and hydroxyacids released by sequential treatments of suspended particles and sediments of the Black Sea, Organic Geochemistry, 30: 1059-1074
http://dx.doi.org/10.1016/S0146-6380(99)00084-4
Zimmerman, A. R., and Canuel, E. A., 2001, Bulk organic matter and lipid biomarker composition of ChesapeakeBay surficial sediments as indicators of environmental processes.Estuarine, Coastal and Shelf Science, 53: 819-341
http://dx.doi.org/10.1006/ecss.2001.0815
. PDF(588KB)
. HTML
Associated material
. Readers' comments
Other articles by authors
. Al-Timari A.Ak
. Al-Saad H.T
. Douabul A.A
. Salah M
. Hantoosh A.A
Related articles
. Fatty acids
. Sediment
. Gas Chromatography
. Shatt Al-Arab
. NW Arabian Gulf
Tools
. Email to a friend
. Post a comment
.jpg)
.png)


